- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Statins improve Graves' orbitopathy in active eye disease with hypercholesterolemia: Lancet
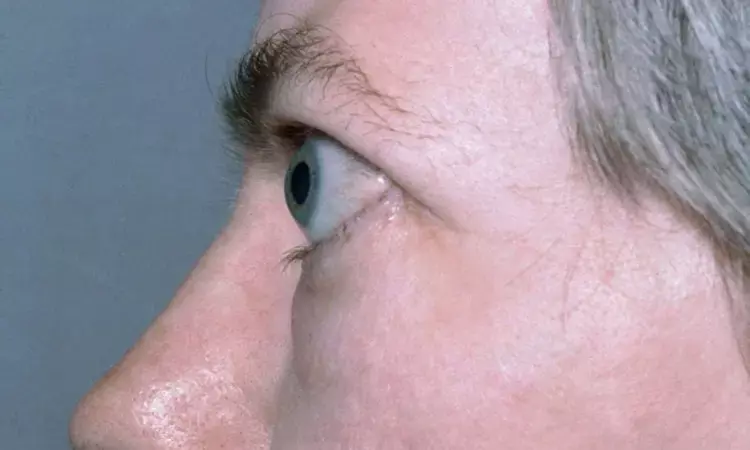
Italy: A recent study has pointed that oral atorvastatin addition to an intravenous glucocorticoids (ivGC) regimen improves the outcomes of Graves' orbitopathy in patients with moderate-to-severe, active eye disease who are hypercholesterolemia. The study appears in The Lancet Diabetes & Endocrinology.
However, according to the researchers, there is a need to confirm this association by conducting phase 3 studies in the future that could potentially recruit patients regardless of low-density lipoprotein cholesterol concentration.
Statins are known to play a protective role in the development of Graves' orbitopathy suggesting that statin might be used for the disease treatment. To determine its use in the treatment, Giulia Lanzolla, University of Pisa and University Hospital of Pisa, Pisa, Italy, and colleagues aimed to assess the efficacy of the addition of a statin, atorvastatin, to ivGCs on Graves' orbitopathy outcomes in patients with hypercholesterolemia.
For achieving their objective, the researchers designed a randomized, open-label, phase 2, adaptive, clinical trial at a single, tertiary, referral hospital in Pisa, Italy. The study included patients with moderate-to-severe, active Graves' orbitopathy, with a low-density lipoprotein cholesterol concentration between 2·97 and 4·88 mmol/L. Thye were randomly assigned in the ratio of 1:1 in 11 blocks of eight to the ST group or the NST group. The ST group received ivGCs (methylprednisolone 500 mg once a week for 6 weeks followed by 250 mg once a week for an additional six weeks) for 12 weeks and oral atorvastatin (20 mg once a day) for 24 weeks. The NST group only received the ivGC regimen.
The primary endpoint was the Graves' orbitopathy outcome (composite evaluation of exophthalmos, clinical activity score, eyelid aperture, and diplopia) at 24 weeks in the modified intention-to-treat (ITT) population (patients who attended the week 12 visit).
Patients were considered responders when at least two of the following criteria were fulfilled in the most affected eye, without worsening in any of the same measures in both eyes: (1) reduction in exophthalmos of 2 mm or more, with no increase by 2 mm or more in the other eye; (2) reduction of clinical activity score by two or more points; (3) reduction in eyelid aperture by 2 mm or more, with no increase by 2 mm or more in the other eye; and (4) disappearance or improvement (change from constant to inconstant, intermittent, or absent, or from inconstant to intermittent or absent) of diplopia, and (5) improvement in visual acuity by 0·2 decimals or more.
88 (74%) patients were enrolled and randomly assigned to one of the two treatment groups (44 [50%] to the ST group and 44 [50%] to the NST group.
Based on the study, the researchers found the following:
- Eight (9%) patients did not attend the 12-week visit; 80 (91%) patients (18 [23%] men and 62 [78%] women) were included in the modified ITT population (41 [51%] in the ST group and 39 [49%] in the NST group].
- The proportion of Graves' orbitopathy composite evaluation responders at 24 weeks was higher in the ST group (21 [51%] of 41 patients) than the NST group (11 [28%] of 39 patients; attributable risk 0·23).
- 26 adverse events occurred in 21 (24%) of 88 patients in the safety population.
- One (2%) of 44 patients in each group required treatment discontinuation, with no serious adverse events and no difference between groups.
"Addition of oral atorvastatin to an ivGC regimen improved Graves' orbitopathy outcomes in patients with moderate-to-severe, active eye disease who were hypercholesterolemia," the researchers wrote.
"Future phase 3 studies, which could potentially recruit patients regardless of low-density lipoprotein cholesterol concentration, are required to confirm this association," they concluded.
Reference:
The study titled, "Statins for Graves' orbitopathy (STAGO): a phase 2, open-label, adaptive, single centre, randomised clinical trial," is published in The Lancet Diabetes & Endocrinology.
DOI: https://www.thelancet.com/journals/landia/article/PIIS2213-8587(21)00238-2/fulltext
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


