- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Topical lipoic acid choline ester eye drops improve near visual acuity; study finds
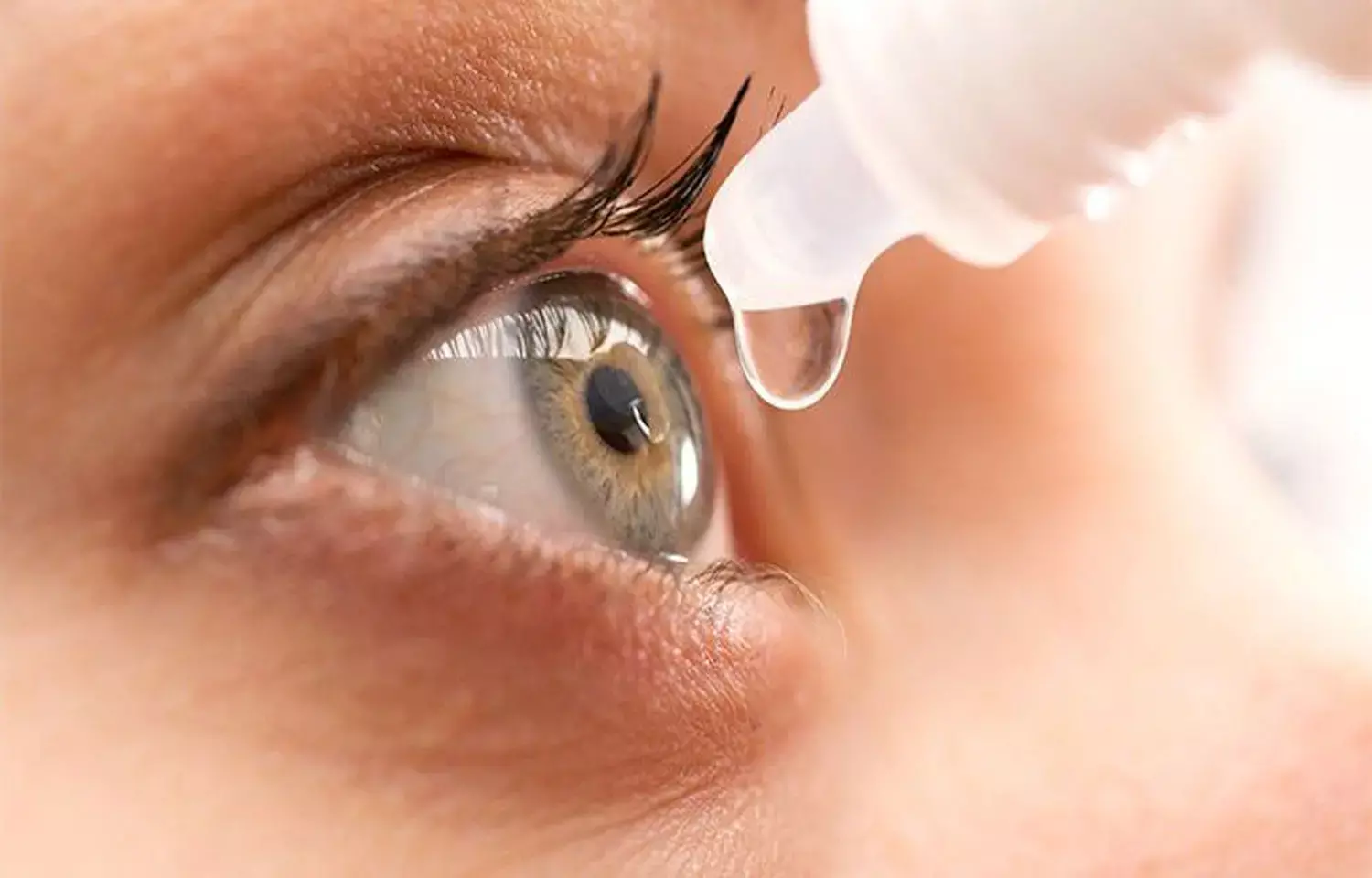
Presbyopia is caused by a combination of ciliary body, vitreous body, and crystalline lens degeneration. The degenerative changes in the crystalline lens are thought to be caused by changes in the flexibility of the lens capsule and its contents, as well as changes in the overall size and shape of the lens.
Lipoic acid (LA) is an antioxidant that has been found to diminish lens disulfide linkages chemically. In preclinical experiments, topical LA enhanced lens flexibility in vitro in a dose-dependent manner. A new research backs up the further development of UNR844 ophthalmic solution for presbyopia therapy.
This study was conducted by Michael S. Korenfeld and team with the objective look at the safety and efficacy of a topical lipoic acid choline ester (UNR844, 1.5 %) ocular solution in enhancing distance-corrected near visual acuity (DCNVA) in people with presbyopia. The findings of this study were published in the Journal of Eye.
This was a multicenter, prospective, randomized, double-masked clinical experiment. Subjects with presbyopia (n = 75) were randomly assigned to either UNR844 or a placebo. On days 1–7, all individuals were dosed unilaterally (twice a day, b.i.d.) in their non-dominant eye to confirm safety and acceptability before switching to bilateral dosage on days 8–91. (b.i.d.). At each trial visit, clinical evaluations, including DCNVA and adverse events (AEs), were documented. Patients who completed the study were enrolled in a non-interventional follow-up study that tracked them for 7 months after their last UNR844 exposure. Safety and the mean change in DCNVA from baseline in the study eye were the main objectives.
The key findings of this study are:
1. UNR844 treatment (n = 50) resulted in no safety issues and was well tolerated, with no clinically significant changes in best-corrected distance visual acuity, pupil size, intraocular pressure, or discontinuations owing to adverse events.
2. During the 91-day treatment period, DCNVA improved in the UNR844 group compared to placebo [UNR844 vs. placebo, mean change in LogMAR (SD); 0.159 (0.120) vs. 0.079 (0.116)].
3. Bilateral DCNVA increased, with 53.1% of UNR844 individuals receiving 10 letters compared to 21.7% of placebo subjects. DCNVA improvements were maintained 5 and 7 months after UNR844 dosage was discontinued.
In conclusion Authors said that this study reveals that UNR844 has the potential to be a first-in-class disease-modifying pharmacological therapy for presbyopia, for which no authorized pharmacological therapeutic options are presently available. A significant unmet need exists for a simple and safe topical ocular therapy option for presbyopia. This study's phase 1/2 results demonstrate UNR844 to be a well-tolerated, effective pharmacological intervention for presbyopia, bolstering the case for continued development of this treatment strategy.
Reference:
Korenfeld MS, Robertson SM, Stein JM, et al. Topical lipoic acid choline ester eye drop for improvement of near visual acuity in subjects with presbyopia: a safety and preliminary efficacy trial. Eye (Lond). 2021;35(12):3292-3301. doi:10.1038/s41433-020-01391-z
Medical Dialogues consists of a team of passionate medical/scientific writers, led by doctors and healthcare researchers. Our team efforts to bring you updated and timely news about the important happenings of the medical and healthcare sector. Our editorial team can be reached at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


