- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Twice-daily lotilaner ophthalmic solution effective treatment of patients with Demodex blepharitis
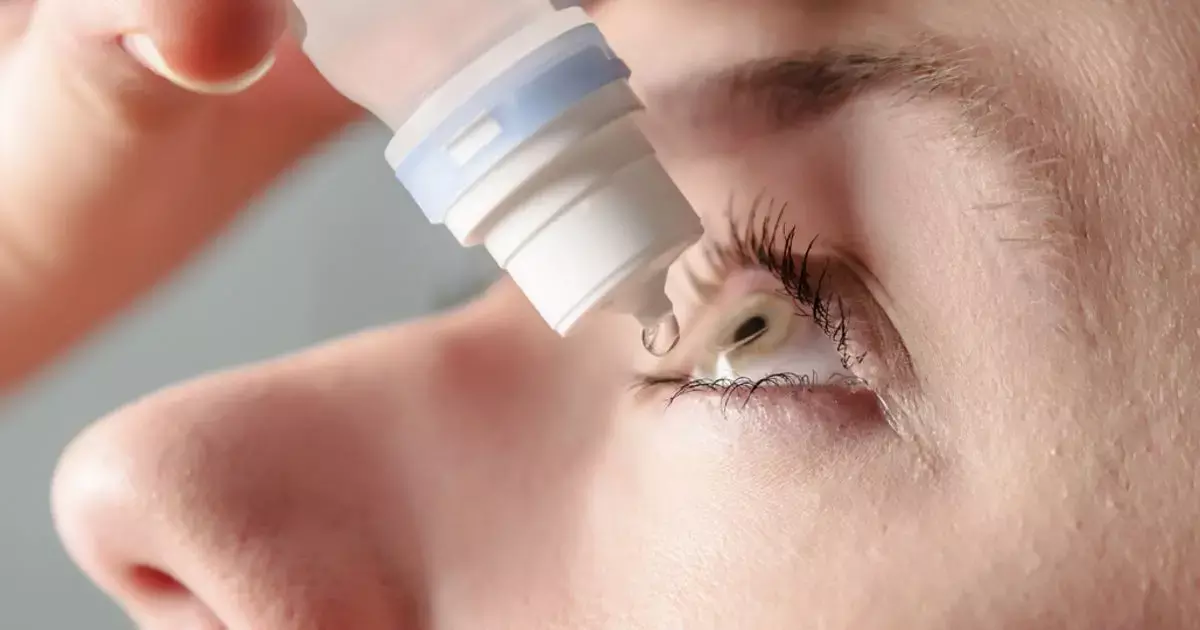
Twice-daily treatment with lotilaner ophthalmic solution,holds promise for the effective treatment of patients with Demodex blepharitis suggests a new study published in the Opthalmology.
A study was done to evaluate the safety and efficacy of lotilaner ophthalmic solution, 0.25% compared to the vehicle for the treatment of Demodex blepharitis.
Four hundred and twelve (412) Demodex blepharitis patients were randomly assigned in a 1:1 ratio to receive either lotilaner ophthalmic solution, 0.25% (study group) or vehicle without lotilaner (control group).
In this study, conducted at 21 U.S. clinical sites, Demodex blepharitis patients assigned to the study group (N=203) received lotilaner ophthalmic solution, 0.25% and those in the control group (N=209) received vehicle without lotilaner bilaterally, twice daily for six weeks. Patients were evaluated on Days 8, 15, 22, and 43. Collarettes and erythema were graded for each eyelid at screening and at all post-baseline visits. At screening, and on Days 15, 22, and 43, 4 or more eyelashes were epilated from each eye, and the number of Demodex mites present on the lashes was counted with a microscope. Mite density was calculated as the number of mites per lash.
Outcome measures included collarette cure (collarette grade 0), clinically meaningful collarette reduction to ≤10 collarettes (grade 0 or 1), mite eradication (0 mites/lash), erythema cure (grade 0), composite cure (grade 0 for collarettes as well as erythema), compliance with the drop regimen, drop comfort, and adverse events.
Results
At Day 43, the study group achieved a statistically significant (p<0.0001) higher proportion of patients with collarette cure (56.0% vs 12.5%), clinically meaningful collarette reduction to ≤10 collarettes (89.1% vs 33.0%), mite eradication (51.8% vs 14.6%), erythema cure (31.1% vs 9.0%), and composite cure (19.2% vs 4.0%) than the control group. High compliance with the drop regimen (mean ± standard deviation: 98.7 ± 5.3%) in the study group was observed, and 90.7% of patients found the drops to be neutral to very comfortable.
Twice-daily treatment with lotilaner ophthalmic solution, 0.25% for 6 weeks was generally safe and well tolerated and met the primary endpoint and all secondary endpoints for the treatment of Demodex blepharitis compared to the vehicle control.
Reference:
Ian Benjamin Gaddie, Eric D. Donnenfeld, Paul Karpecki, John Meyer, Elizabeth Yeu, Saturn II Study Group. Lotilaner Ophthalmic Solution, 0.25% for Demodex Blepharitis: Randomized, Vehicle-Controlled, Multicenter, Phase 3 Trial (Saturn-2). Published:June 05, 2023DOI:https://doi.org/10.1016/j.ophtha.2023.05.030
Dr. Shravani Dali has completed her BDS from Pravara institute of medical sciences, loni. Following which she extensively worked in the healthcare sector for 2+ years. She has been actively involved in writing blogs in field of health and wellness. Currently she is pursuing her Masters of public health-health administration from Tata institute of social sciences. She can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


