- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
IM or IA glucocorticoid injection, which is better for reducing pain in knee osteoarthritis?
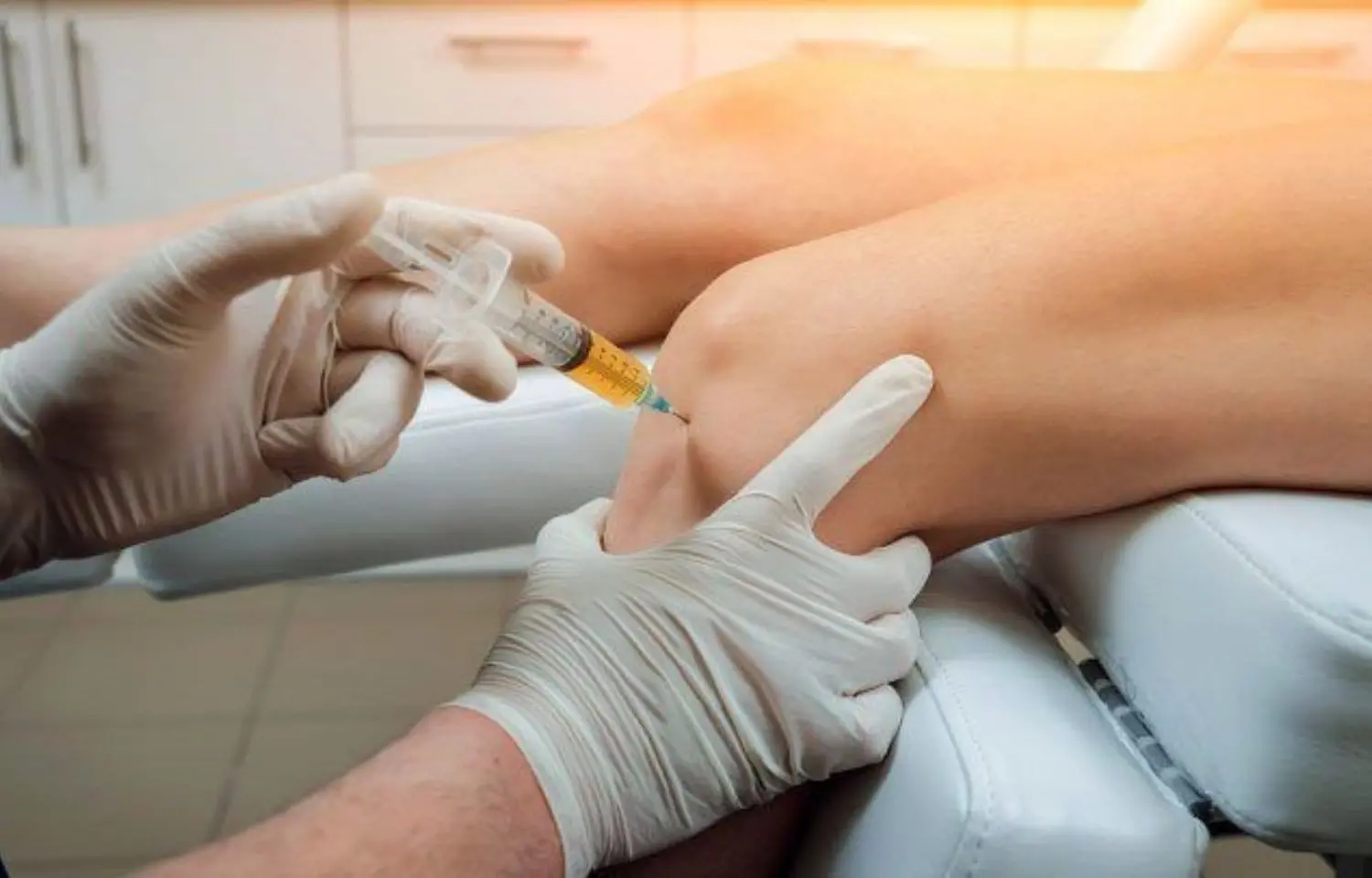
Netherlands: Both intramuscular (IM) glucocorticoid injection and intra-articular (IA) glucocorticoid injection should be considered effective strategies for patients with knee osteoarthritis (OA), suggests findings from a trial. And, a shared decision-making process should take place between clinicians and knee OA patients in cases when a glucocorticoid injection is indicated. The study appears in JAMA Network Open.
"IM glucocorticoid injection could present an inferior effect in reducing pain at 4 weeks compared with IA injection in patients with knee OA in primary care," Qiuke Wang, Erasmus MC University Center Rotterdam, Rotterdam, the Netherlands, and colleagues wrote in their study. "Noninferiority of an IM injection was seen at 8 and 24 weeks after injection. The trial provides data for shared decision-maling considering the advantages and disadvantages of injection types."
IA glucocorticoid injection is widely used in knee OA patients but the safety of this technique is questionable. An alternative approach could be IM glucocorticoid injection. The study therefore was conducted to investigate whether an IM glucocorticoid injection is noninferior to an IA glucocorticoid injection in reducing knee pain for patients with knee OA in primary care in KIS trial.
KIS is a multicenter, open-label, randomized clinical noninferiority trial. It included patients with symptomatic knee OA and was conducted in 80 primary care general practices in the southwest of the Netherlands. The study was conducted from March 1, 2018, to July 28, 2020.
Patients were randomly allocated to receive an injection of triamcinolone acetonide, 40 mg, either IM in the ipsilateral ventrogluteal region or IA in the knee joint and were followed for 24 weeks.
The pain score at 4 weeks were measured with Knee Injury and Osteoarthritis Outcome Score (range, 0-100; 0 indicates extreme pain). A per-protocol analysis was prespecified as the primary analysis.
Of 145 patients included in the trial; 138 patients (IM, 72; IA, 66) were included in the per-protocol analysis.
Based on the findings, the researchers reported the following:
- Clinically relevant improvements in knee pain were reached up to 12 weeks after the injection in both groups. At 4 weeks, the estimated mean difference in the Knee Injury and Osteoarthritis Outcome Score between the 2 groups was −3.4.
- Noninferiority could not be declared because the lower limit exceeded the noninferiority margin.
- Intramuscular injection was noninferior to IA injection at 8 (mean difference, 0.7) and 24 (mean difference, 1.6) weeks.
- No significant difference was found among all the secondary outcomes. These results were similar for the sensitivity analysis in an intention-to-treat population.
- The most frequently reported adverse events were hot flush (IM, 7 [10%] vs IA, 14 [21%]) and headache (IM, 10 [14%] vs IA, 12 [18%]), and all events were classified as nonserious.
The authors concldued, "Both types of injection should be considered effective strategies, and this trial provides evidence for shared decision-making between clinicians and patients, taking into account the advantages and disadvantages of both treatment strategies."
Reference:
Wang Q, Mol MF, Bos PK, et al. Effect of Intramuscular vs Intra-articular Glucocorticoid Injection on Pain Among Adults With Knee Osteoarthritis: The KIS Randomized Clinical Trial. JAMA Netw Open. 2022;5(4):e224852. doi:10.1001/jamanetworkopen.2022.4852
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


