- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Mavacamten provides sustained improvement of HOCM symptoms even beyond 1 year: VALOR-HCM trial
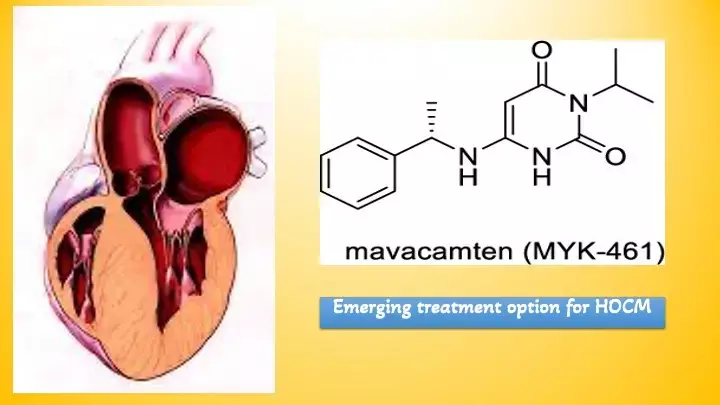
The first drug in class of cardiac myosin inhibitors , mavacamten, received US FDA approval in 2022 following results of the EXPLORER-HCM trial. At the same time, the longer-term efficacy of mavacamten in reducing the need for septal reduction therapy (SRT) in patients with obstructive hypertrophic cardiomyopathy (HCM) is not known.
Desai et al in a recent extended follow-up study of VALOR-HCM trial have now shown that for patients with symptomatic obstructive HCM, there is sufficient and sustained improvement with mavacamten at 56 weeks of follow-up, thereby reducing the need for SRT and representing a useful therapeutic option for patients.
To examine the cumulative longer-term effect of mavacamten on the need for SRT through week 56, the authors recruited patients from 19 US HCM centers. Included in the trial were patients with obstructive HCM (NYHA class III/IV) referred for SRT.
Patients initially assigned to mavacamten at baseline continued the drug for 56 weeks, and patients taking placebo crossed over to mavacamten from week 16 to week 56 (40-week exposure). Dose titrations were performed using echocardiographic LVOT gradient and LV ejection fraction (LVEF) measurements.
The efficacy end point was the composite of a decision to proceed with SRT or eligibility for SRT as per AHA guidelines.
Findings of the trial:
1. Among 112 patients with highly symptomatic obstructive HCM in the original VALOR-HCM trial, 108 qualified for the week 56 evaluation.
2. At week 56, 8.9% in the original mavacamten group and 19.2% in the placebo crossover group met the composite end point.
3. Around 90% patients continued mavacamten long term. Between the mavacamten and placebo-to-mavacamten groups, respectively, after 56 weeks, there was a sustained reduction in resting and Valsalva LVOT gradients.
4. Similarly, there was an improvement in NYHA class of 1 or higher in 93% in the original mavacamten group and 73% in the placebo crossover group.
5. Overall, 12 of 108 patients had an LVEF less than 50% but 9 out of 12 continued treatment.
To summarise, at week 56, there was a sustained therapeutic effect, with only 6 patients (5.6%) undergoing SRT and 89% remaining in the long-term extension phase. The reduction in SRT eligibility, improvement in NYHA class, and LVOT gradients paves way for mavacamten to be a useful therapeutic option for symptomatic HCM patients.
A word of caution:
“It is noteworthy that 11% of patients developed LVEF less than 50% during the course of this study, which underscores the need for careful monitoring and additional long-term safety data”, noted the authors.
Although mavacamten must be used carefully, the ability to defer SRT in most patients for more than 1 year indicates a favorable balance of benefit to risk given the invasive nature of alternative treatments.
Source: JAMA Cardiology: doi:10.1001/jamacardio.2023.3342
MBBS, MD , DM Cardiology
Dr Abhimanyu Uppal completed his M. B. B. S and M. D. in internal medicine from the SMS Medical College in Jaipur. He got selected for D. M. Cardiology course in the prestigious G. B. Pant Institute, New Delhi in 2017. After completing his D. M. Degree he continues to work as Post DM senior resident in G. B. pant hospital. He is actively involved in various research activities of the department and has assisted and performed a multitude of cardiac procedures under the guidance of esteemed faculty of this Institute. He can be contacted at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


