- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Postapproval study results of Impella show no mortality concerns: FDA
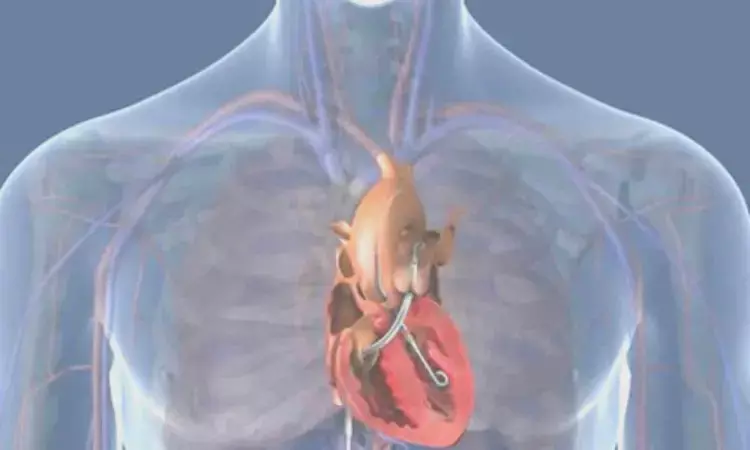
USA: In its recently released update, the U.S. Food and Drug Administration (FDA) has said that final postapproval study (PAS) results for the Impella RP system now alleviate concerns about a potential mortality signal previously seen with the percutaneous device. Also, the FDA updated the labelling for Abiomed's Impella RP System.
The final results from the PAS revealed that the survival rate for the subgroup of PAS patients who would have met the enrollment criteria for the premarket clinical studies is consonant with the premarket clinical studies survival rate. The findings further confirmed that the device is safe and effective when used for the currently approved indication.
Based on the PAS results, the FDA approved updated labelling for the Impella RP System on December 5, 2022, with an update of the indications for use statement to better consider the characteristics of the patients who may benefit the most from the device's treatment.
The results showed a survival rate of 69.7% for the PAS patients who would have been eligible for enrollment in premarket clinical studies. Survival was defined as living to 30 days hospital discharge or post explant or to receipt of a surgically implanted right ventricular assist device or heart transplant.
However, the survival rate for those who would not have met enrollment criteria was 18.6%. In these patients, the PAS survival rate should be interpreted in the context of conditions and limited treatment options. Such patients, according to the FDA, were more likely to have been in acute right heart failure or decompensation for longer than 48 hours and experienced a severe cardiogenic shock, end-organ failure, or acute neurologic injury.
"The Impella RP System is indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open‐heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury," the FDA wrote in its statement.
The FDA believes that when the device is used for the currently approved indication in appropriately selected patients the benefits of the Impella RP System continue to outweigh the risks.
The FDA encourages health care providers to give information on any adverse events or suspected adverse events associated with the use of Impella RP System. "Prompt reporting of adverse events can help the FDA identify and better understand the risks associated with medical devices," the article stated.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


