- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Tapinarof shows promise in Plaque Psoriasis treatment in phase 3 trial results
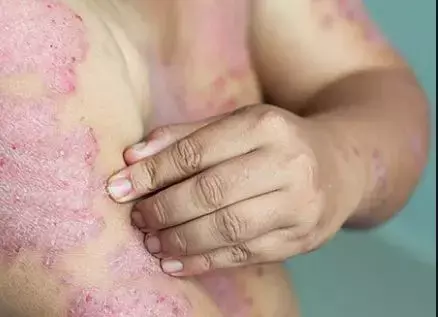
Researchers have reported positive results with the use of Tapinarof cream (Dermavant Sciences), from 2 identical phase 3 studies.
Both studies were published in The Journal of the American Academy of Dermatology (JAAD), the official, peer-reviewed, scientific publication of the American Academy of Dermatology (AAD).
Psoriasis is a chronic, systemic, inflammatory skin disease characterized by red patches and plaques with silvery scales on the skin.
Tapinarof is a novel aryl hydrocarbon receptor modulating agent (TAMA), for the treatment of plaque psoriasis in adult patients. The most commonly reported AEs were folliculitis, nasopharyngitis, and contact dermatitis.
With this as background, a group of researchers undertook the PSOARING 1 and PSOARING 2 studies. They were two identical, multi-center, randomized, vehicle-controlled, double-blind, parallel studies undertaken to evaluate the efficacy and safety of tapinarof cream, 1% in adult patients with plaque psoriasis.
The study population collectively enrolled 1,025 patients, who were treated with 1% dosed QD for 12 weeks versus vehicle QD ,in adult patients aged 18-75 years diagnosed with plaque psoriasis. Adult patients with plaque psoriasis (510 in PSOARING 1 and 515 in PSOARING 2) were randomized in a 2:1 ratio to receive once daily (QD) treatment with tapinarof cream, 1% or vehicle cream.
The primary endpoint of both studies was a PGA score of clear (0) or almost clear (1) with a minimum 2-grade improvement from baseline at Week 12.
Following the 12-week, a vehicle-controlled portion of PSOARING 1 and PSOARING 2, patients had the option to enroll in PSOARING 3, a separate, long term, open-label extension study for an additional 40 weeks of treatment. Greater than 90% of eligible patients who completed PSOARING 1 and PSOARING 2 enrolled in the long-term safety study.
Results of the trials showed that-
· In both PSOARING 1 (N=510) and PSOARING 2 (N=515), tapinarof cream demonstrated highly statistically significant improvement in Physician Global Assessment (PGA) score of clear (0) or almost clear (1) with a minimum 2-grade improvement compared to the vehicle from baseline at Week 12 (both P<0.0001), thus meeting the primary endpoint of each trial.
· Additionally, in both trials, tapinarof cream demonstrated a highly statistically significant improvement in PASI75 from baseline at Week 12 (both P<0.0001), a key secondary endpoint.
· Also, up to 80% of patients achieved a ≥1-grade improvement in PGA across both studies.
"Based on previously published data and my firsthand experience as an investigator, this highly statistically significant Phase 3 results point to tapinarof as an effective potential new treatment for psoriasis," said Mark G. Lebwohl, MD, Dean for Clinical Therapeutics at the Icahn School of Medicine at Mount Sinai, and the lead investigator for the PSOARING 1 study.
As the trials are ongoing, detailed results are being awaited for.
For the full article, click the link: https://www.businesswire.com/news/home/20200826005389/en/Dermavant-Reports-Positive-Phase-3-Results-Tapinarof.
Dr Satabdi Saha (BDS, MDS) is a practicing pediatric dentist with a keen interest in new medical researches and updates. She has completed her BDS from North Bengal Dental College ,Darjeeling. Then she went on to secure an ALL INDIA NEET PG rank and completed her MDS from the first dental college in the country – Dr R. Ahmed Dental College and Hospital. She is currently attached to The Marwari Relief Society Hospital as a consultant along with private practice of 2 years. She has published scientific papers in national and international journals. Her strong passion of sharing knowledge with the medical fraternity has motivated her to be a part of Medical Dialogues.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


