- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
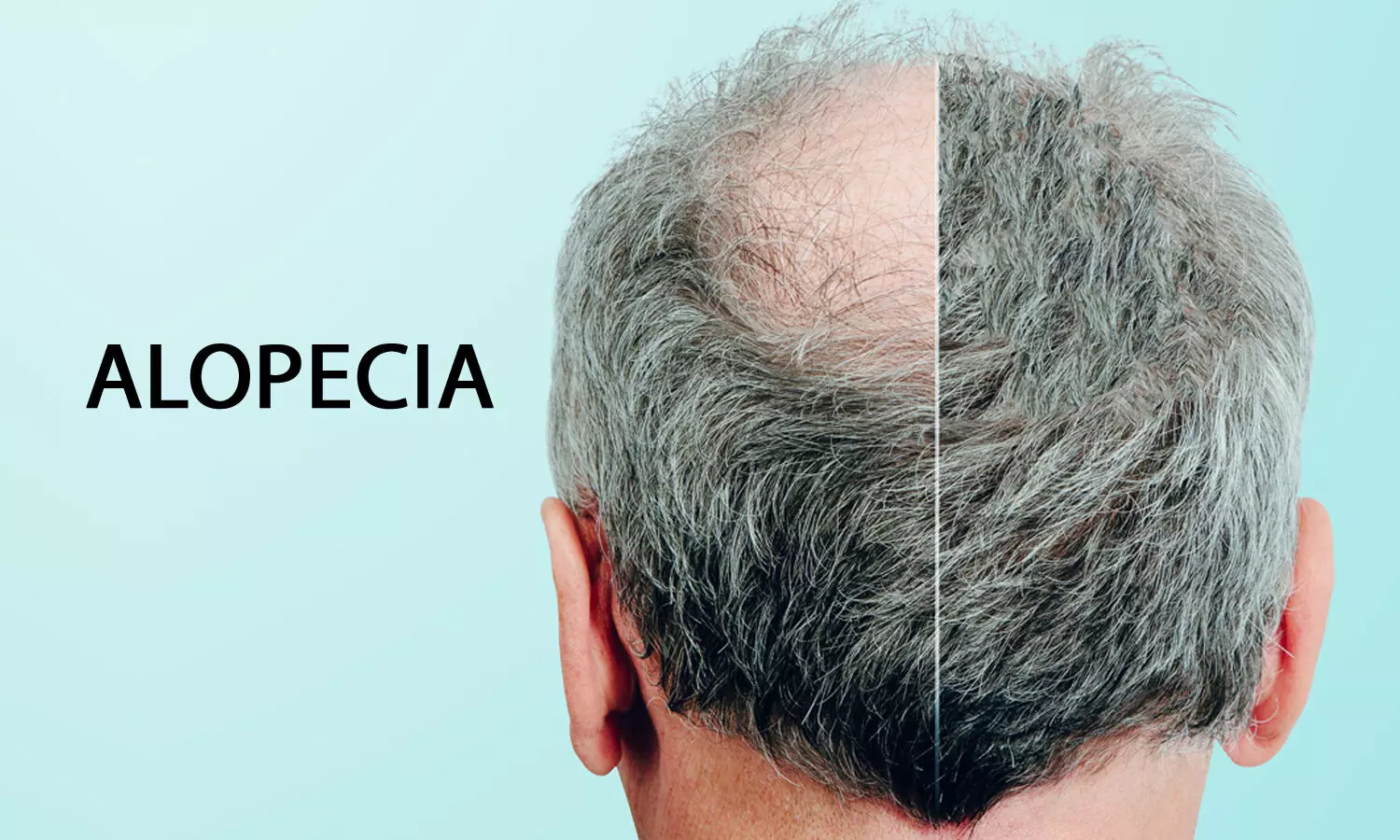
Dr Reddys Minoxidil gets CDSCO panel nod to treat androgenic alopecia in females

New Delhi: The Subject Expert Committee (SEC) of the Central Drugs Standard Control Organization (CDSCO) has given its nod to pharma major Dr Reddy's Laboratories for manufacturing and marketing Minoxidil Topical Solution 2% and Minoxidil Topical solution 5% for the treatment of androgenic alopecia in females with female pattern baldness.
The approval came in the wake of the proposal presented by Dr. Reddy's laboratories for manufacturing and marketing of Minoxidil Topical Solution 2% & 5% for the treatment of androgenic alopecia in females with female pattern baldness with a request for Clinical Trial waiver.
Androgenetic alopecia is a genetically predetermined disorder due to an excessive response to androgens. This condition is characterized by progressive loss of terminal hair on the scalp any time after puberty. This condition affects up to 50 percent of males and females.
Minoxidil and finasteride are the only two medicines currently authorised by the US Food and Drug Administration (FDA) for the treatment of androgenetic alopecia.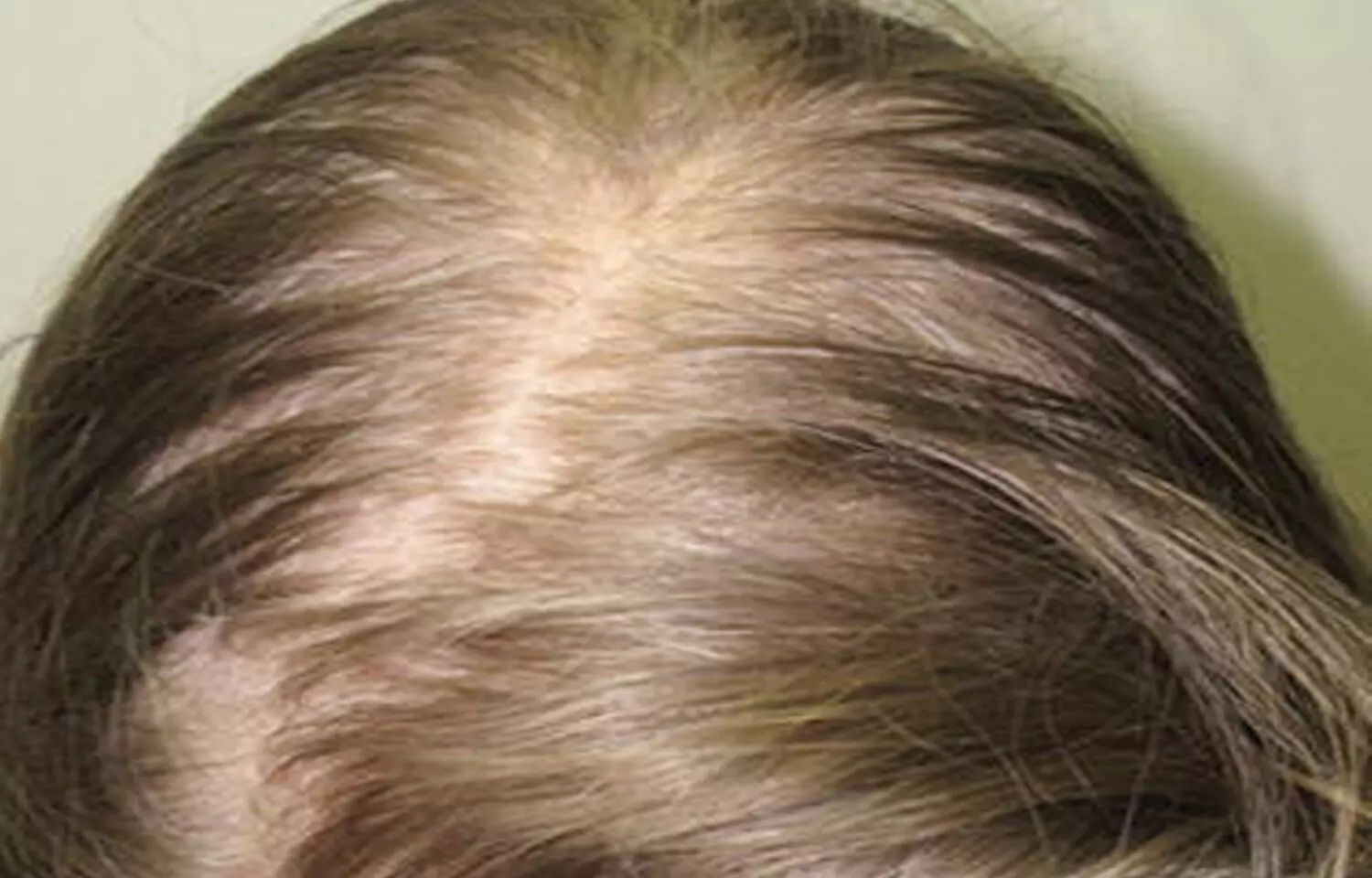
In the 1970s, minoxidil was launched as an oral drug to treat severe and resistant hypertension. It is an orally administered vasodilator with hair growth stimulatory and antihypertensive effects.
Minoxidil is converted into its active metabolite minoxidil sulphate by sulphotransferase enzymes. Minoxidil sulphate exerts its antihypertensive effect by opening plasma membrane adenosine triphosphate (ATP)-sensitive potassium channels (KATP channels), thereby directly and rapidly relaxing arteriolar smooth muscle and subsequent reduction of elevated systolic and diastolic blood pressure by decreasing peripheral vascular resistance.
Minoxidil's hair growth stimulatory effect may be mediated through its vasodilatory activity, thereby increasing cutaneous blood flow, or due to its direct stimulatory effect on hair follicle cells and forcing them from their resting phase into their active growth phase.
Some popular brands of Minoxidil include Sun Pharma's Exidil 5% Solution 60ml, Amexidil-5 Solution 60ml, Dr. Reddy's Mintop Forte 5% Foam 60gm, CIPLA's Tugain 5% Gel 60gm etc.
At the 60th SEC meeting for Dermatology & Allergy held on 12.08.2021, the committee thoroughly reviewed the proposal presented by Dr. Reddy's laboratories for manufacturing and marketing of Minoxidil Topical Solution 2% & 5% for the treatment of androgenic alopecia in females with female pattern baldness with a request for a Clinical Trial waiver.
After detailed deliberation, the committee recommended the grant of permission for the manufacturing and marketing of Minoxidil Topical Solution 2% & 5% for the treatment of androgenic alopecia in females (female pattern hair loss).