- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
'Insulin Tablets' banned, License of pharma firm cancelled after activist flags mislabeling risks for diabetics
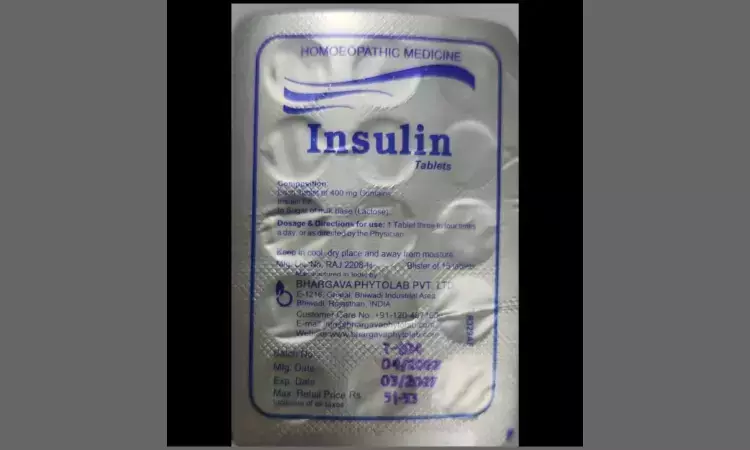
New Delhi: The license of Bhargava Phytolabs, a Rajasthan-based pharmaceutical company, has been cancelled for manufacturing "Insulin Tablets," a homeopathic product, the Prime Minister’s Office (PMO) said in response to an RTI.
This decision follows a complaint filed by Kerala-based RTI activist Dr K.V. Babu, who claimed that the manufacture and labelling of these tablets violated Section 106 A(C) of the Drugs and Cosmetics Rules, 1945.
Background of the Issue
Dr. K.V. Babu, an ophthalmologist known for his activism in healthcare, initially raised concerns on January 24 by filing a formal complaint with the Drugs Controller General of India (DCGI). Dr Babu urged the agency to take action against Bhargava Phytolabs for allegedly breaching regulations under the Drugs and Cosmetics Act, 1940, and the associated Drugs and Cosmetics Rules, 1945. His primary concern centered around Section 106 A(C), which mandates that single-ingredient homeopathic medications should not carry proprietary or brand names on their labels. This regulation aims to ensure that product labels are transparent, straightforward, and devoid of misleading proprietary names, especially for single-ingredient drugs like homeopathic Insulin 6x.
The complaint emphasized that the labelling could potentially mislead consumers, who might assume the product functions identically to traditional insulin used for managing diabetes. Insulin is a drug for patients with type-1 diabetes and sometimes type-2 diabetes, is crucial for preventing complications such as coronary artery disease, stroke, and other vascular conditions.
The concern was that patients could mistake the homeopathic “Insulin Tablets” as a viable alternative to medically prescribed insulin, potentially leading to severe health risks, particularly for diabetic children dependent on insulin injections for blood sugar regulation.
Government Response and Regulatory Investigation
After Dr Babu’s initial complaint, the Central Drugs Standard Control Organisation (CDSCO) launched a review into the licensing of Bhargava Phytolabs’ product. In June, a comprehensive examination by the Union Ayush Ministry revealed that the company had inaccurately labelled its product. The Ministry found that Bhargava Phytolabs labelled the product as “Insulin Tablets,” implying it was a proprietary product when, in fact, it was a single-ingredient homeopathic remedy (Insulin 6x). The labelling breach highlighted that the company had branded the tablets, contravening the specific labelling rules outlined in the Drugs and Cosmetics Rules, 1945.
Misleading Implications and Potential Health Risks
Dr Babu expressed concerns that the labelling of the product as “Insulin Tablets” could mislead patients. He feared that patients, particularly those unfamiliar with the distinctions between homeopathic and allopathic treatments, might switch from essential insulin injections to these tablets, believing them to serve the same purpose. This risk was especially pressing for diabetic children and others managing serious blood glucose issues, as substituting homeopathic tablets for insulin injections could result in dangerously uncontrolled diabetes.
In response to these regulatory findings, Bhargava Phytolabs argued in May that their product had obtained proper licensing from the Rajasthan State Drug Licensing Authority. The Ayush Ministry, however, determined that this licensing was based on inaccurate labelling practices and that the product should instead be labelled as “Insulin Homoeopathic trituration tablets” with clear potency information. This renaming would align with regulatory standards, thus reducing the likelihood of public confusion.
RTI Activism and PMO Intervention
The situation reached a critical point when Dr Babu escalated his concerns to the Prime Minister’s public grievance cell on September 23. In his appeal, Dr Babu emphasized the potential dangers associated with misleading labelling practices and urged the PMO to intervene to protect public health. The PMO took cognizance, formally responding on October 23 that Bhargava Phytolabs had not applied for the renewal of its license for the “Insulin Tablets.” Consequently, the PMO confirmed that the license for this product had been officially cancelled.
In its response, the PMO on October 23 said, “The grievance submitted by the applicant is regarding the violation of Rule 106A (C) of Drug Rules, 1945 w.r.t. Insulin Tablets, Homeopathic proprietary medicine. In this regard, the matter has already been communicated to the Drugs Controller (Rajasthan), and a response has been received. It has stated that the manufacturing licencee has not submitted the application for the renewal of the licence for the said product. Hence, the licence for the said product is cancelled.”
Speaking to Medical Dialogues, Dr Babu said, “My concern was that a tablet being made available with the name insulin may confuse the patients, who may switch over from insulin injections to these tablets. This could lead to uncontrolled diabetes, especially in children. I approached the PM’s grievance cell as the State Drugs Controller of Rajasthan and the manufacturer refused to budge even after the CDSCO and the Union Ayush Ministry made their stand clear about the illegal labelling.”
"I am happy that the license issued to "insulin tablets" stands cancelled," Babu added.
Farhat Nasim joined Medical Dialogue an Editor for the Business Section in 2017. She Covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She is a graduate of St.Xavier’s College Ranchi. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751


