- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Meril Life Sciences unveils indigenously developed bioresorbable scaffold MeRes100 in India
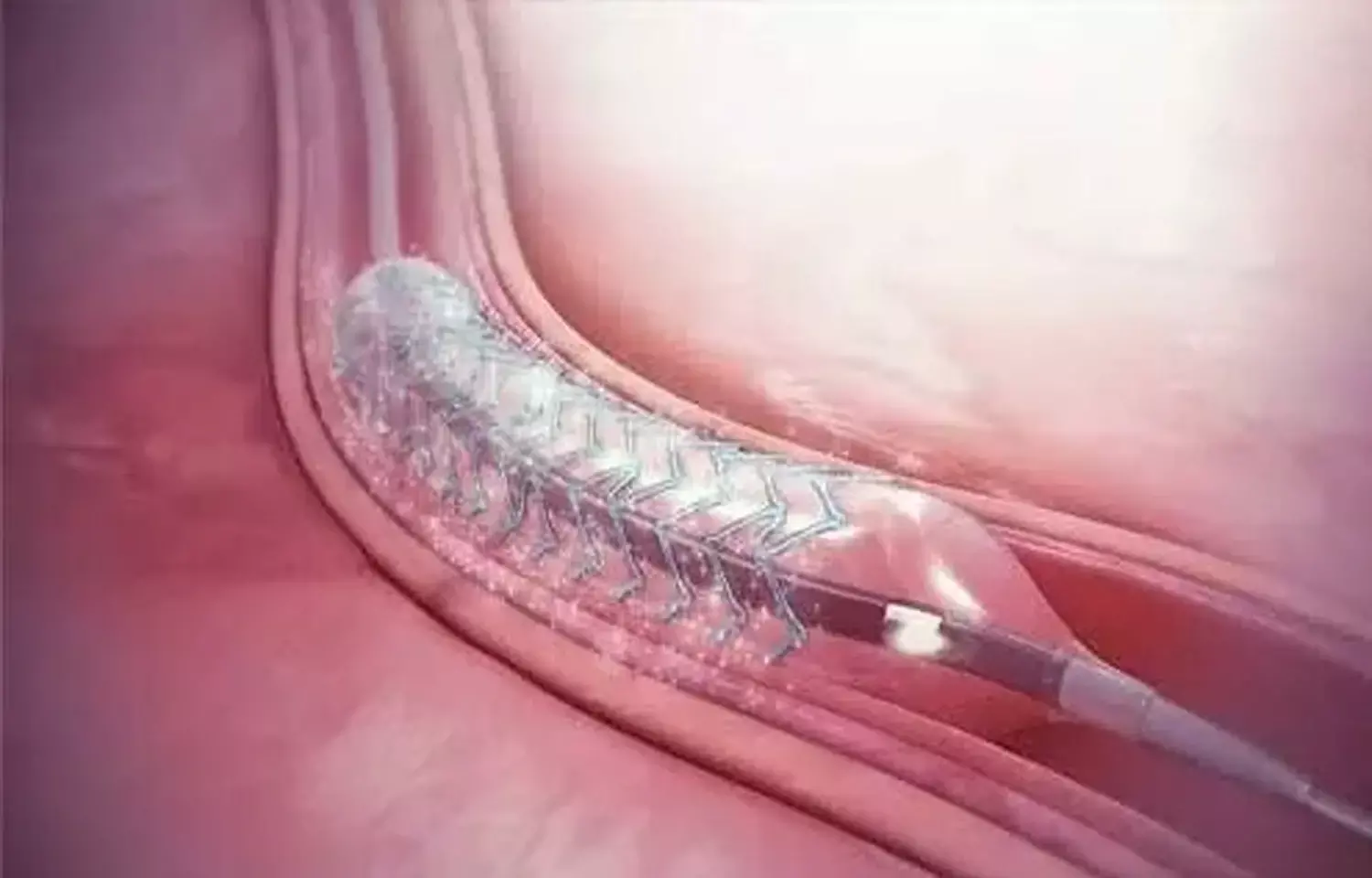
New Delhi: Meril Life Sciences on Wednesday said it has launched indigenously researched and developed bioresorbable scaffold (BRS) MeRes100 in the country.
Bioresorbable scaffolds are non-metallic, non-permanent mesh tubes, similar to stents, that dissolve over time after ensuring the previously blocked artery is opened via a routine angioplasty procedure.
This novel therapy option, which can meaningfully treat an identified subset of the patient population, will be launched in a phased, sequential manner to ensure adherence to best clinical practices and continued development of clinical research and long-term evidence, Meril Life Sciences said in a statement.
Currently, MeRes100 is being launched in 16 cities across the country -- including Mumbai, Delhi, Gurgaon, Bangalore, Chennai, Pune, Hyderabad, Ahmedabad, Lucknow, Chandigarh, Mohali, Jaipur, Kochi and Eddakad (in Kerala), Nagpur and Bhubaneswar.
"We are committed to introduce MeRes100 BRS in India and ensure it is delivered to the right patients in line with the right indication.
"We will ensure sequential hospital roll-out to facilitate best clinical practices, appoint a team of trained clinical specialists to assist cases and conduct doctor education on protocols for successful bioresorbable stent implantation," Meril Life Sciences Corporate Strategy Senior Vice President Sanjeev Bhatt said.
Through these measures, the company aims to facilitate the best outcomes for patients, he added.
MeRes100 is the first-ever 100 micron thin-strut BRS developed to treat people with coronary artery disease. Till date, it has been granted a total of 12 patents from USA, Japan, Australia, Russia, Europe, Korea, China, Brazil and India, Meril Life Sciences said.
MeRes100 has received approvals from DCGI and the European regulator.
Ruchika Sharma joined Medical Dialogue as an Correspondent for the Business Section in 2019. She covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She has completed her B.Com from Delhi University and then pursued postgraduation in M.Com. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751


