- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
No casual link found between GLP-1 drugs like Novo Nordisk Ozempic, thyroid cancer: EMA
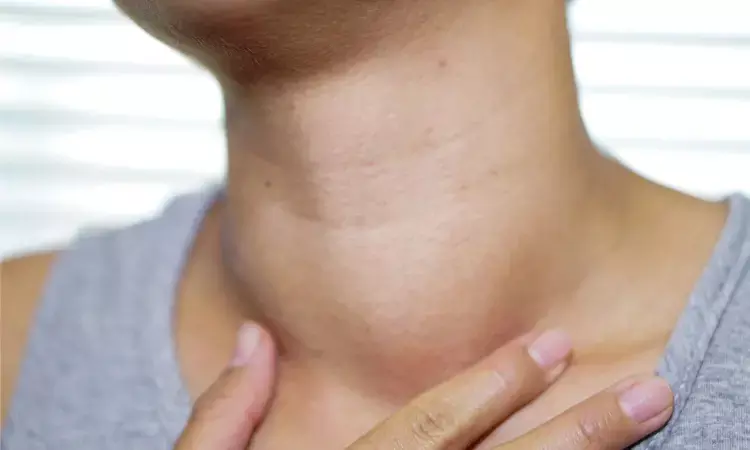
The European Medicines Agency (EMA) has said its safety panel did not find a causal link between popular GLP-1 drugs such as Novo Nordisk's Ozempic and thyroid cancer after a months-long review.
The panel committee reviewed non-clinical, clinical and post-marketing data of the drugs, and said that no updates to their prescribing information was required at the time.
The agency has since April been monitoring the use of GLP-1 receptor agonists, a class of diabetes and weight loss drugs that includes Ozempic and Wegovy, for any signs that they increase the risk of thyroid cancer.
The safety panel reviewed data from Novo's popular diabetes drug Ozempic and weight loss treatment Wegovy, which contain the same active ingredient, semaglutide.
It also reviewed data from the Danish drugmaker's other diabetes drug Victoza and obesity drug Saxenda, which contain liraglutide.
Novo's U.S. rival Eli Lilly's diabetes drug Trulicity, or dulaglutide, and British rival AstraZeneca's Bydureon, or exenatide, was also part of the review.
The panel said that manufacturers of these drugs should continue to monitor safety events closely and report any new data to the regulator.
In July, the regulator also began reviewing a group of GLP-1 drugs after Iceland's health regulator flagged three cases of patients thinking about suicide or self-harm after using Novo's medications.
GLP-1 receptor agonists, originally developed to help control blood sugar in patients with diabetes, also slow digestion and reduce hunger.
Mounjaro, a similar diabetes medicine from Eli Lilly, is also being used off-label as a weight loss medication.
Read also: Novo Nordisk to stop once-weekly injectable semaglutide kidney outcomes trial
Ruchika Sharma joined Medical Dialogue as an Correspondent for the Business Section in 2019. She covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She has completed her B.Com from Delhi University and then pursued postgraduation in M.Com. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751


