- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
FDA clears PENS device for relief of post-op pain of cesarean section
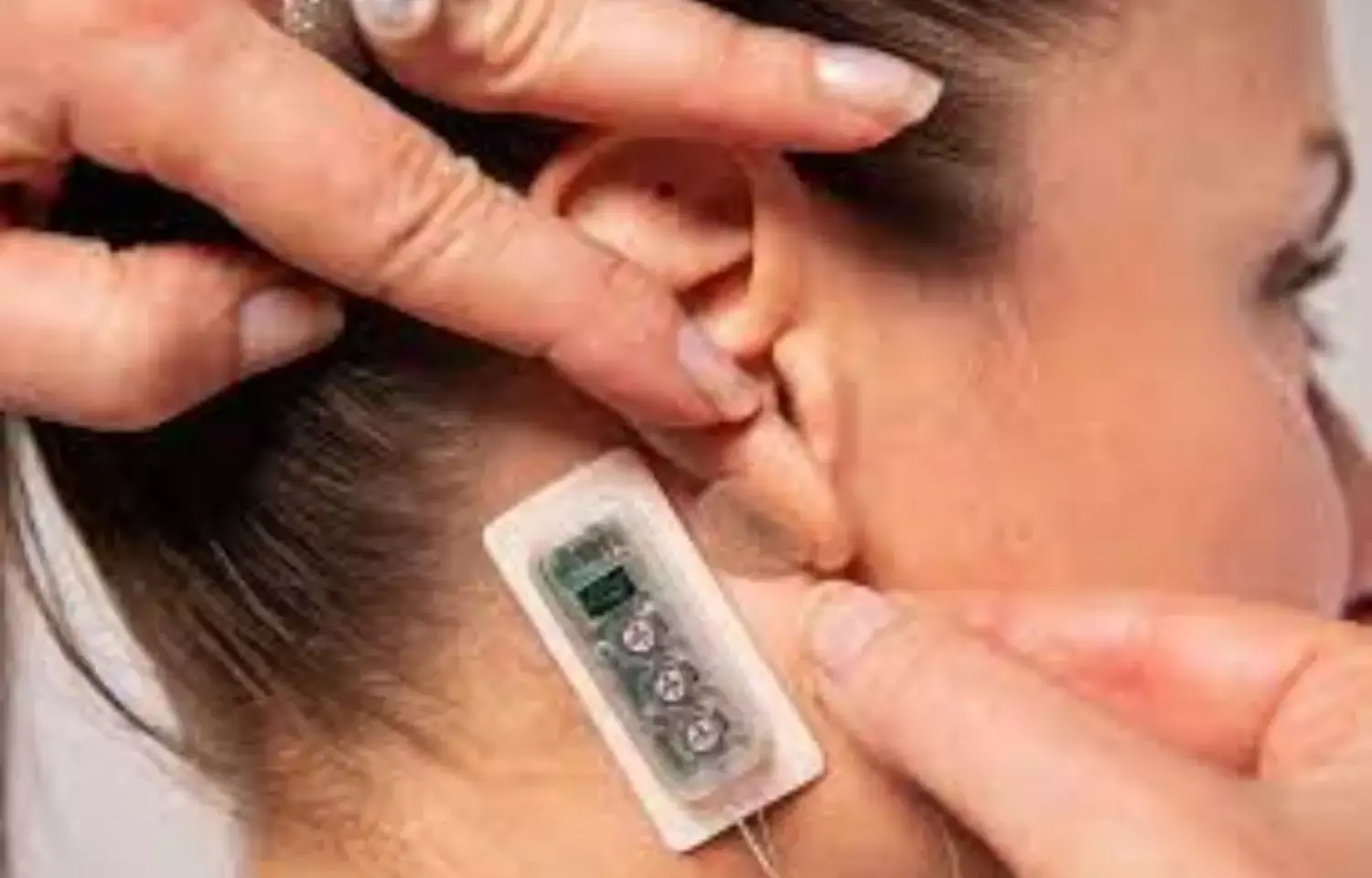
The US Food and Drug Administration has cleared a novel percutaneous electrical neurostimulation (PENS) device Primary Relief for symptomatic relief of postoperative pain following caesarean section delivery.
The manufacturing company DyAnsys, Inc., has announced that Primary Relief, a percutaneous electrical neurostimulation device, has been cleared to treat pain following a Caesarean-section (C-section).
The percutaneous electrical nerve stimulator (PENS) system can be used for up to three days for symptomatic relief of post-operative pain following a C-section delivery.
"This device has been shown to make a difference for patients, effectively relieving pain without reliance on opioids or other analgesics. This is a significant advancement in providing options to women," said DyAnsys Chief Executive Srini Nageshwar. "We look forward to connecting with physicians and hospitals to providing this alternative to their patients."
The wearable, battery-operated device is designed to administer periodic low level electrical pulses to the ear over 72 hours from the activation of the device. The electrical pulses are delivered to branches of the cranial nerves on the ear through a wire assembly and stimulation needles.
The effectiveness of the device was demonstrated in a single-center, double arm, randomized, controlled, parallel assignment prospective study involving 44 participants who underwent C-section delivery.
The analysis showed that minimally invasive nerve stimulation intervention using Primary Relief device reduced the pain score faster than the standard analgesics. No additional opioid analgesics were required. No complications or adverse events were observed in any of the participants during the study period.
The nonclinical testing of Primary Relief device included biocompatibility testing, electrical safety (electromagnetic compatibility and safety), performance bench testing and software verification and validation.
References
FDA clears DyAnsys neurostimulation device primary relief to treat C-section pain. News release. DyAnsys, Inc. Accessed July 28, 2022. https://www.prnewswire.com/news-releases/fda-clears-dyansys-neurostimulation-device-primary-relief-to-treat-c-section-pain-301595255.html
Primary Relief 510(k) summary. Food and Drug Administration. Accessed July 28, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf21/K213188.pdf
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


