- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
PHMB monotherapy effective alternative to PHMB and propamidine combo for treatment of Acanthamoeba keratitis
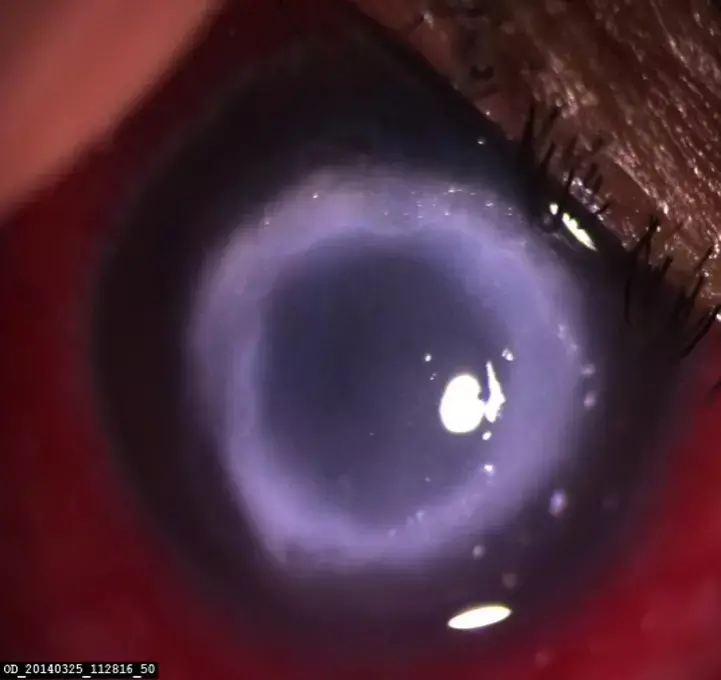
Acanthamoeba keratitis (AK) is a rare but serious eye infection caused by a microscopic amoeba and typically requires complex treatments. A recent Phase 3 clinical trial has shown that PHMB 0.08% (0.8 mg/ml) alone may be as effective as dual therapy with PHMB 0.02% (0.2 mg/ml) + propamidine 0.1% (1 mg/ml) for treating Acanthamoeba keratitis (AK).
This study was published in Ophthalmology by John Dart and colleagues. This was a prospective, randomised, double-masked, active-controlled, multicenter Phase 3 study. The study involved 135 participants aged 12 or older with clinical findings consistent with AK. Some participants had concurrent bacterial keratitis and had used other eye medications before the study. Participants were randomly assigned to receive either PHMB 0.02% + propamidine or PHMB 0.08%. The primary outcome was the MCR_12, defined by clinical criteria 90 days after discontinuing anti-inflammatories and anti-amoebic therapy. A prespecified multivariable analysis was conducted to adjust for baseline imbalances in risk factors affecting outcomes. The primary outcome, MCR_12, was assessed for both treatment groups, and secondary outcomes included BCVA and treatment failure rates. Safety outcomes focused on adverse event rates.
Results:
- A total of 135 participants were randomized.
- The adjusted MCR_12 was 86.6% for PHMB 0.02% + propamidine and 86.7% for PHMB 0.08%.
- The non-inferiority requirement for PHMB 0.08% was met.
- Secondary outcomes, including median BCVA of 20/20 and treatment failure rates, were similar for both treatments.
- No serious drug-related adverse events were reported.
The study's primary outcome, the medical cure rate (MCR_12) within 12 months, demonstrated that both treatment groups had MCRs exceeding 86%, meeting the non-inferiority requirement for PHMB 0.08%. Secondary outcomes, including best-corrected visual acuity (BCVA) and treatment failure rates, were similar for both treatments. Importantly, no serious drug-related adverse events were reported.
This study suggests that PHMB 0.08% monotherapy may be a viable treatment option for AK, demonstrating effectiveness comparable to dual therapy. The findings provide hope for more straightforward and cost-effective treatments for this rare but challenging eye condition.
Reference:
Dart, J. K. G., Papa, V., Rama, P., Knutsson, K. A., Ahmad, S., Hau, S., Sanchez, S., Franch, A., Birattari, F., Leon, P., Fasolo, A., Kominek, E. M., Jadczyk-Sorek, K., Carley, F., Hossain, P., & Minassian, D. C. The Orphan Drug for Acanthamoeba Keratitis (ODAK) trial: PHMB (polihexanide) 0.08% and placebo versus PHMB 0.02% and propamidine 0.1%. Ophthalmology,2023. https://doi.org/10.1016/j.ophtha.2023.09.031
Dr Riya Dave has completed dentistry from Gujarat University in 2022. She is a dentist and accomplished medical and scientific writer known for her commitment to bridging the gap between clinical expertise and accessible healthcare information. She has been actively involved in writing blogs related to health and wellness.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


