- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Phase 2 Outcomes of novel agent Shows Promise Against Tophaceous Gout
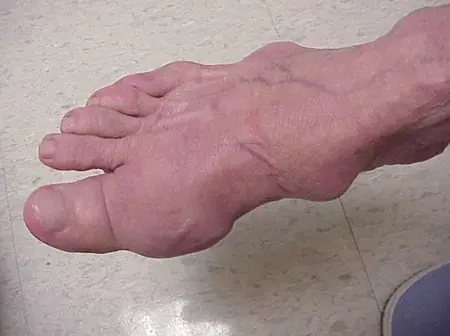
The novel URAT1 inhibitor, AR882 exhibited robust and sustained serum urate (sUA) reduction in t Phase 2b trial in treating gout and tophaceous gout. The trial published in American College of Rheumatology evaluated AR882 against the conventional treatment allopurinol in reducing clinically visible tophi in gout patients.
This study involved 42 gout patients with subcutaneous tophi who were randomly assigned to three groups receiving AR882 75 mg, AR882 50 mg + allopurinol, or allopurinol up to 300 mg. Over a period of six months, caliper measurements and Dual Energy Computer Tomography (DECT) were utilized to monitor tophi reduction. The primary endpoint was the change in sUA levels at month 3, with secondary endpoints including resolution of the target tophus area and changes in tophus crystal volume at month 6.
Results demonstrated a remarkable reduction in mean sUA levels across all groups, with the 75 mg AR882 group outperforming others, achieving sUA levels < 6 and < 5 mg/dL in 86% and 64% of patients, respectively.
The AR882 groups exhibited fewer gout flares compared to the allopurinol group. At month 6, AR882 showed complete resolution of at least one tophus in 29% of patients in the 75 mg group, highlighting its efficacy in tophus reduction.
AR882 demonstrated a good safety profile with no serious adverse events related to the treatment. The most frequently reported adverse event was mild gout flare, and no abnormalities were observed in kidney or liver function.
This findings suggest that AR882 may provide some improved and safer alternative for gout treatment. This in turn offers hope to patients with both clinically visible and subclinical crystal deposition. Since this study represents a critical step in the drug's development, the findings pave the way for further investigation, positioning AR882 as a potential game-changer in the management of gout and tophaceous gout.
Source:
Keenan R, WEI J, Morris S, Mundell P, Wei W, Shi K, Shen Z, Hingorani V, Yan S, Kiani B, Yeh L. AR882, an efficacious and selective URAT1 inhibitor for patients with chronic gouty arthritis and subcutaneous tophi: Results from a global, prospective, proof-of-concept trial using dual energy computed tomography. (2023, October 17). ACR Meeting Abstracts.
Neuroscience Masters graduate
Jacinthlyn Sylvia, a Neuroscience Master's graduate from Chennai has worked extensively in deciphering the neurobiology of cognition and motor control in aging. She also has spread-out exposure to Neurosurgery from her Bachelor’s. She is currently involved in active Neuro-Oncology research. She is an upcoming neuroscientist with a fiery passion for writing. Her news cover at Medical Dialogues feature recent discoveries and updates from the healthcare and biomedical research fields. She can be reached at editorial@medicaldialogues.in
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


