- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Aroxybutynin and atomoxetine combo good treatment option for sleep apnea in patients with low CPAP adherence
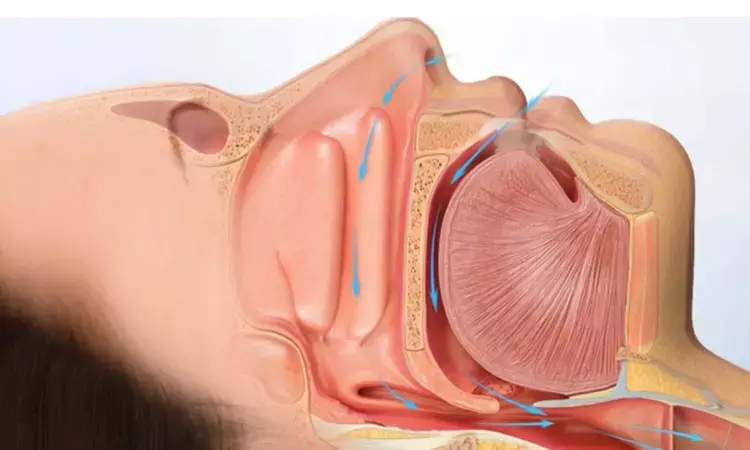
A recent Phase II clinical trial has unveiled promising results for the treatment of obstructive sleep apnea (OSA) with AD109, a novel combination medication comprising aroxybutynin, an antimuscarinic agent, and atomoxetine, a norepinephrine reuptake inhibitor. This study was published in the American Journal Of Respiratory And Critical Care Medicine by Paula K. and colleagues.
This randomized, double-blind, placebo-controlled study, spanning four weeks and involving 211 participants, evaluated the efficacy and safety of AD109 in comparison with atomoxetine alone and placebo. Patients were assessed using polysomnograms to gauge sleep parameters, with the primary outcome being the reduction in the apnea–hypopnea index (AHI) with a 4% desaturation criterion, a key indicator of OSA severity.
The results demonstrated compelling improvements in sleep-related metrics:
Reduction in AHI: The AD109 2.5/75 mg arm showcased a remarkable decrease from a median AHI of 20.5 to 10.8 (−47.1%), while the AD109 5/75 mg arm exhibited a reduction from 19.4 to 9.5 (−42.9%). Notably, both AD109 doses significantly outperformed placebo (P < 0.0001). The atomoxetine alone group also demonstrated a meaningful decrease from 19.0 to 11.8 (−38.8%; P < 0.01 vs. placebo).
Subjective Improvements: Participants reported significant fatigue improvement with AD109 2.5/75 mg (P < 0.05 vs. placebo and atomoxetine). However, atomoxetine alone was associated with reduced total sleep time (P < 0.05 vs. AD109 and placebo).
The safety profile of AD109 was favorable, with the most common adverse events including dry mouth, insomnia, and urinary hesitancy. The study's findings suggest that AD109 could offer clinically meaningful benefits for individuals with OSA, warranting further investigation and development of this compound as a potential pharmacotherapy for OSA. The novel combination of aroxybutynin and atomoxetine has shown promise in addressing OSA symptoms, potentially providing a new avenue for patients who struggle with conventional treatments like continuous positive airway pressure (CPAP).
Reference:
Schweitzer, P. K., Taranto-Montemurro, L., Ojile, J. M., Thein, S. G., Drake, C. L., Rosenberg, R., Corser, B., Abaluck, B., Sangal, R. B., & Maynard, J. The combination of aroxybutynin and atomoxetine in the treatment of obstructive sleep apnea (MARIPOSA): A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine,2023;208(12):1316–1327. https://doi.org/10.1164/rccm.202306-1036oc
Dr Riya Dave has completed dentistry from Gujarat University in 2022. She is a dentist and accomplished medical and scientific writer known for her commitment to bridging the gap between clinical expertise and accessible healthcare information. She has been actively involved in writing blogs related to health and wellness.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


