- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
USFDA approves OxyContin for children aged 11 to 16
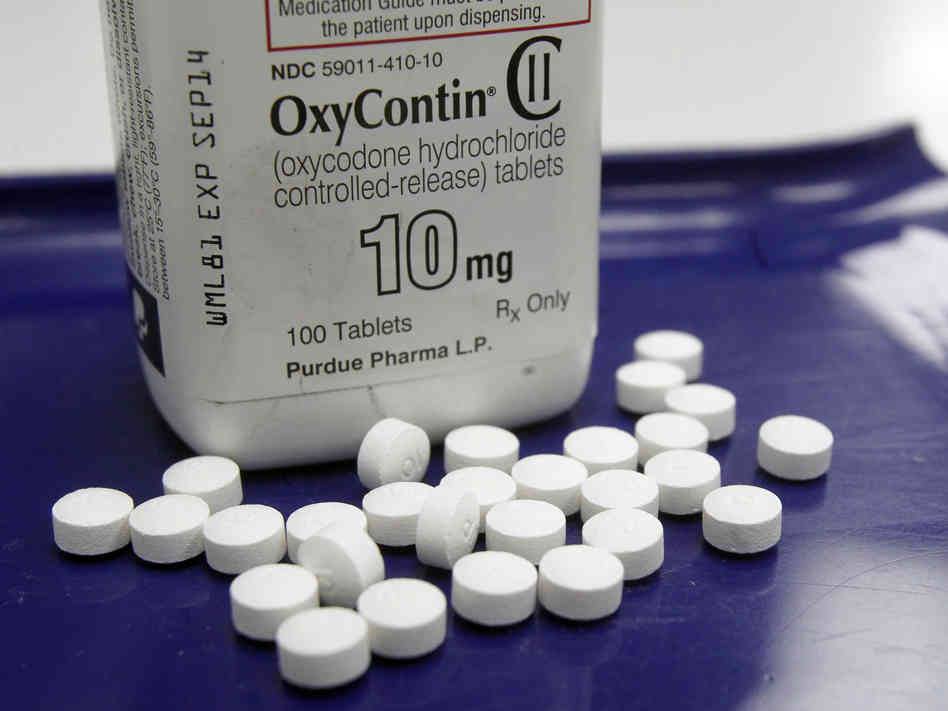
USFDA has approved OxyContin, a narcotic painkiller for children aged 11 to 16. The approval has been received with mixed response, with doctors treating young cancer patients hailing the use of opioid, while others doubting the use of drug on children fearing addiction.
OxyContin is an extended-release opioid that has long been used to treat around-the-clock pain in adults. But most pain medications are not approved for use in children.
The FDA says it asked drugmaker Purdue Pharma to study how to safely use OxyContin in children.
Under the approval announced Thursday, prescribers are directed to only prescribe OxyContin to children who can already tolerate another opioid. Taking a sudden dose of an opioid can lead to overdose and death if patients haven't previously been exposed to the drugs.
The FDA notes that the Duragesic patch is the only other opioid approved for children.
OxyContin is an extended-release opioid that has long been used to treat around-the-clock pain in adults. But most pain medications are not approved for use in children.
The FDA says it asked drugmaker Purdue Pharma to study how to safely use OxyContin in children.
Under the approval announced Thursday, prescribers are directed to only prescribe OxyContin to children who can already tolerate another opioid. Taking a sudden dose of an opioid can lead to overdose and death if patients haven't previously been exposed to the drugs.
The FDA notes that the Duragesic patch is the only other opioid approved for children.
Meghna A Singhania is the founder and Editor-in-Chief at Medical Dialogues. An Economics graduate from Delhi University and a post graduate from London School of Economics and Political Science, her key research interest lies in health economics, and policy making in health and medical sector in the country. She is a member of the Association of Healthcare Journalists. She can be contacted at meghna@medicaldialogues.in. Contact no. 011-43720751
Next Story


