- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Dupilumab Shows Promising Results for Hair Regrowth in Pediatric Alopecia Areata, with No New Safety Concerns: Study
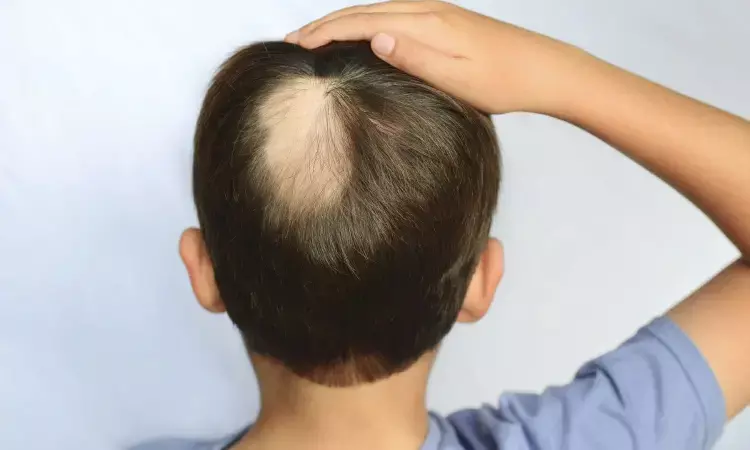
USA: A real-world, single-center observational study revealed that dupilumab significantly enhanced hair regrowth in pediatric patients with alopecia areata (AA) and atopic dermatitis, suggesting it may be a safe and promising long-term treatment option.
"The real-world data affirm the effectiveness of dupilumab for safely treating pediatric patients with alopecia areata (AA), supporting the involvement of Th2 skewing in children with AA and associated atopy. This underscores the need for larger clinical trials to further explore these findings," the researchers wrote in Archives of Dermatological Research.
Alopecia areata is a nonscarring form of hair loss marked by Th1 and concurrent Th2 skewing, especially in atopic individuals. While there have been advances in treating adult AA, safe and effective options for pediatric patients are still lacking. Dupilumab, known for its well-established safety profile, could offer promising therapeutic potential for pediatric patients with atopic alopecia areata.
Against the above background, Emma Guttman-Yassky, Department of Dermatology, and Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York, NY, USA, and colleagues aimed to evaluate the ability of dupilumab to regrow hair in pediatric AA patients.
For this purpose, the researchers conducted a single-center, retrospective observational study to assess hair regrowth using the Severity of Alopecia Tool (SALT) in 20 children aged 5 to 16 years (mean age 10.8 years) with both atopic dermatitis (AD) and alopecia areata (AA). The baseline SALT scores ranged from 3 to 100, with a mean of 54.4.
The study collected data on patient demographics, atopic history, IgE levels, and SALT scores during follow-up visits at 12 weeks and up to more than 72 weeks to evaluate hair regrowth. Spearman correlations were used to analyze the relationship between clinical data and hair regrowth outcomes.
The study led to the following findings:
- Patients showed clinical improvement over the follow-up period (range 24 to > 72 weeks, mean 67.6 weeks) with a significant mean reduction in SALT at 48 weeks versus baseline [20.4 versus 54.4, respectively] and continued improvement up to > 72 weeks.
- Baseline SALT positively correlated with disease duration (r = 0.54), and negatively correlated with improvement in SALT at weeks 24, 36, and 48 (|r|≥ 0.65 for all comparisons).
- Baseline IgE positively correlated with improvement in SALT at week 36 (r > 0.60).
The study has several limitations, including its retrospective design, relatively small sample size, and absence of a comparator group. Despite these constraints, it represents the largest cohort of pediatric alopecia areata patients treated with dupilumab over an extended follow-up period.
"Future research, including larger randomized placebo-controlled trials, is needed to further assess the benefits of dupilumab for the long-term management of AA in children and adolescents with concurrent atopy and familial atopic conditions," the researchers concluded.
Reference:
David, E., Shokrian, N., Del Duca, E. et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res 316, 487 (2024). https://doi.org/10.1007/s00403-024-03225-4
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


