- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Dupilumab receives FDA's Breakthrough Therapy Status for eosinophilic esophagitis
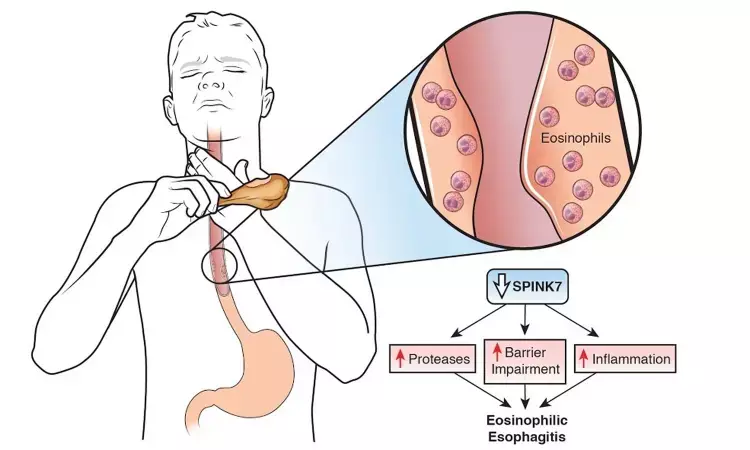
USA: Dupilumab (Dupixent) has received the US FDA's Breakthrough Therapy designation for the treatment of eosinophilic esophagitis (EoE) in patients aged 12 years and older. Breakthrough Therapy designation is designed to expedite the development and review of drugs in the U.S. that target serious or life-threatening conditions.
The designation for this investigational use is based on positive results from Part A of a Phase 3 trial that evaluated Dupixent in patients 12 years and older with EoE. Part A of the randomized, double-blind, placebo-controlled trial of 81 patients met both of its co-primary endpoints, as well as all key secondary endpoints.
Other key findings of the trial include:
- Patients treated weekly with Dupixent 300 mg over a 24-week treatment period experienced a reduction in symptoms, esophageal inflammation and abnormal endoscopic findings in the esophagus.
- The trial demonstrated safety results consistent with the known safety profile of Dupixent in its approved indications.
The EoE trial is ongoing, with additional patients enrolling in Part B as well as patients continuing in a 28-week extended active treatment period (Part C) after completing either Part A or Part B.
Dupixent is the first and only biologic to show positive and clinically-meaningful Phase 3 results in patients 12 years and older with eosinophilic esophagitis.
There are currently no FDA-approved medicines for EoE, a chronic and progressive type 2 inflammatory disease that damages the esophagus and prevents it from working properly. Over time, excessive type 2 inflammation causes scarring and narrowing of the esophagus, making it difficult to swallow. If left untreated, EoE can affect a patient's ability to eat and cause food to become stuck after being swallowed (food impaction), which can lead to a medical emergency.
About Dupixent
Dupixent is an interleukin-4 receptor alpha antagonist indicated:
- for the treatment of adult patients with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. DUPIXENT can be used with or without topical corticosteroids.
- as an add-on maintenance treatment in patients with moderate-to-severe asthma aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid dependent asthma.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


