- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
Risankizumab safe and effective maintenance therapy for Crohn's Disease: Lancet
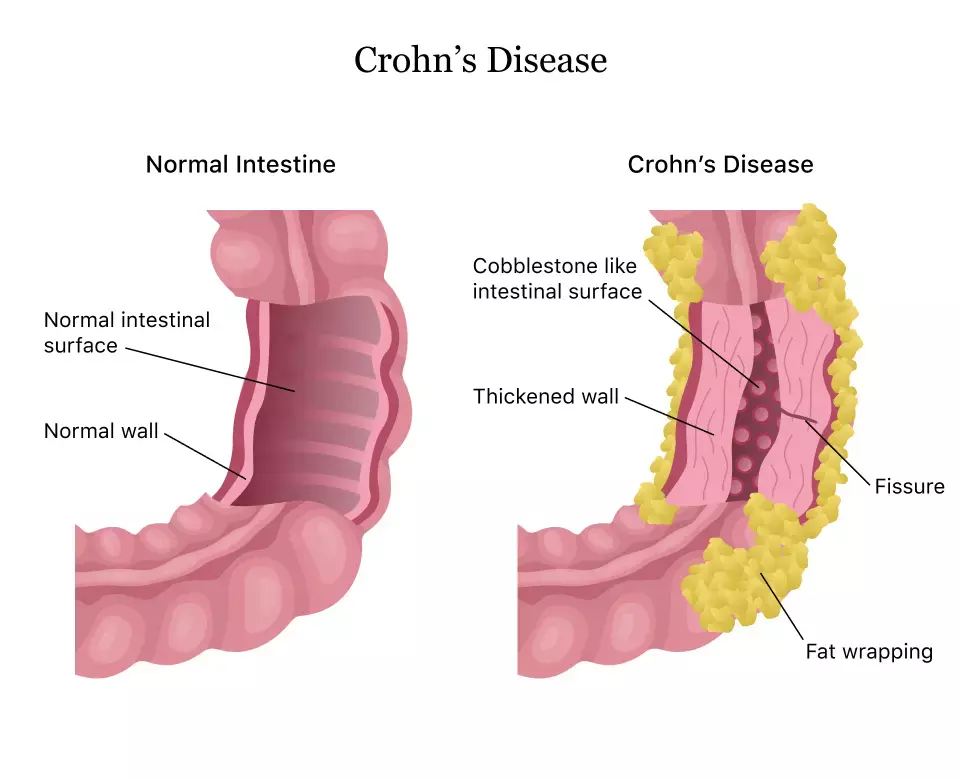
Belgium: A new study conducted by Marc Ferrante and the team showed that subcutaneous risankizumab is a safe and effective treatment for maintaining remission in individuals with moderately to highly active Crohn's disease. The findings of this study were published in The Lancet.
There is a significant unmet demand for new medicines with unique mechanisms of action for Crohn's disease patients. Intravenous risankizumab, a specific p19 anti-interleukin (IL)-23 antibody, was found to be effective and well-tolerated as induction treatment in the ADVANCE and MOTIVATE investigations. The purpose of this research was to evaluate the effectiveness and safety of subcutaneous risankizumab as a treatment modality.
FORTIFY is a phase 3 multicenter, double-blind, randomized, placebo-controlled, maintenance withdrawal trial that included people with a clinical response to risankizumab in the ADVANCE or MOTIVATE induction studies in 44 countries throughout North and South America, Europe, Oceania, Africa, and the Asia-Pacific region.
Patients in the ADVANCE or MOTIVATE groups ranged in age from 16 to 80 years old and had moderately to severely active Crohn's disease. Patients in the FORTIFY sub-study 1 were randomly allocated (1:1:1) to obtain either subcutaneous risankizumab 180 mg, subcutaneous risankizumab 360 mg, or risankizumab withdrawal for a subcutaneous placebo. Every 8 weeks, treatment was administered. Patients were divided into three groups based on their induction dosage, post-induction endoscopic reaction, and clinical remission status.
Treatment assignments were concealed from patients, investigators, and research employees. Clinical remission and endoscopic response were co-primary objectives at week 52 in participants who received at least one dose of trial medication during the 52-week maintenance period. Patients who received at least one dosage of trial medicine were evaluated for safety.
The key findings of this study were as follows:
1. 712 individuals were first evaluated, and between April 9, 2018, and April 24, 2020, 542 people were randomly allocated to one of three groups: risankizumab 180 mg (n=179), risankizumab 360 mg (n=179), or placebo (n=184).
2. When compared to placebo, 360 mg risankizumab achieved higher rates of clinical remission and endoscopic response.
3. Risankizumab 180 mg also resulted in higher percentages of CDAI clinical remission and endoscopic response compared to placebo.
4. The findings for more rigorous endoscopic and composite endpoints, as well as inflammatory biomarkers, supported a dose-response association.
5. The maintenance therapy was well tolerated.
6. Adverse event rates were comparable across groups, with worsening Crohn's disease, arthralgia, and headache being the most commonly reported adverse events across all treatment groups.
In conclusion, the findings of this study point out that it is a novel therapeutic option for a wide spectrum of patients by satisfying endpoints that may modify the course of illness in the future.
Reference:
Ferrante, M., Panaccione, R., Baert, F., Bossuyt, P., Colombel, J.-F., Danese, S., Dubinsky, M., Feagan, B. G., Hisamatsu, T., Lim, A., Lindsay, J. O., Loftus, E. V., Jr, Panés, J., … D'Haens, G. (2022). Risankizumab as maintenance therapy for moderately to severely active Crohn's disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. In The Lancet (Vol. 399, Issue 10340, pp. 2031–2046). Elsevier BV. https://doi.org/10.1016/s0140-6736(22)00466-4
Medical Dialogues consists of a team of passionate medical/scientific writers, led by doctors and healthcare researchers. Our team efforts to bring you updated and timely news about the important happenings of the medical and healthcare sector. Our editorial team can be reached at editorial@medicaldialogues.in.
Dr Kamal Kant Kohli-MBBS, DTCD- a chest specialist with more than 30 years of practice and a flair for writing clinical articles, Dr Kamal Kant Kohli joined Medical Dialogues as a Chief Editor of Medical News. Besides writing articles, as an editor, he proofreads and verifies all the medical content published on Medical Dialogues including those coming from journals, studies,medical conferences,guidelines etc. Email: drkohli@medicaldialogues.in. Contact no. 011-43720751


