- Home
- Medical news & Guidelines
- Anesthesiology
- Cardiology and CTVS
- Critical Care
- Dentistry
- Dermatology
- Diabetes and Endocrinology
- ENT
- Gastroenterology
- Medicine
- Nephrology
- Neurology
- Obstretics-Gynaecology
- Oncology
- Ophthalmology
- Orthopaedics
- Pediatrics-Neonatology
- Psychiatry
- Pulmonology
- Radiology
- Surgery
- Urology
- Laboratory Medicine
- Diet
- Nursing
- Paramedical
- Physiotherapy
- Health news
- Fact Check
- Bone Health Fact Check
- Brain Health Fact Check
- Cancer Related Fact Check
- Child Care Fact Check
- Dental and oral health fact check
- Diabetes and metabolic health fact check
- Diet and Nutrition Fact Check
- Eye and ENT Care Fact Check
- Fitness fact check
- Gut health fact check
- Heart health fact check
- Kidney health fact check
- Medical education fact check
- Men's health fact check
- Respiratory fact check
- Skin and hair care fact check
- Vaccine and Immunization fact check
- Women's health fact check
- AYUSH
- State News
- Andaman and Nicobar Islands
- Andhra Pradesh
- Arunachal Pradesh
- Assam
- Bihar
- Chandigarh
- Chattisgarh
- Dadra and Nagar Haveli
- Daman and Diu
- Delhi
- Goa
- Gujarat
- Haryana
- Himachal Pradesh
- Jammu & Kashmir
- Jharkhand
- Karnataka
- Kerala
- Ladakh
- Lakshadweep
- Madhya Pradesh
- Maharashtra
- Manipur
- Meghalaya
- Mizoram
- Nagaland
- Odisha
- Puducherry
- Punjab
- Rajasthan
- Sikkim
- Tamil Nadu
- Telangana
- Tripura
- Uttar Pradesh
- Uttrakhand
- West Bengal
- Medical Education
- Industry
CDSCO shows green flag to Mylan for remdesivir for 'restricted emergency use'
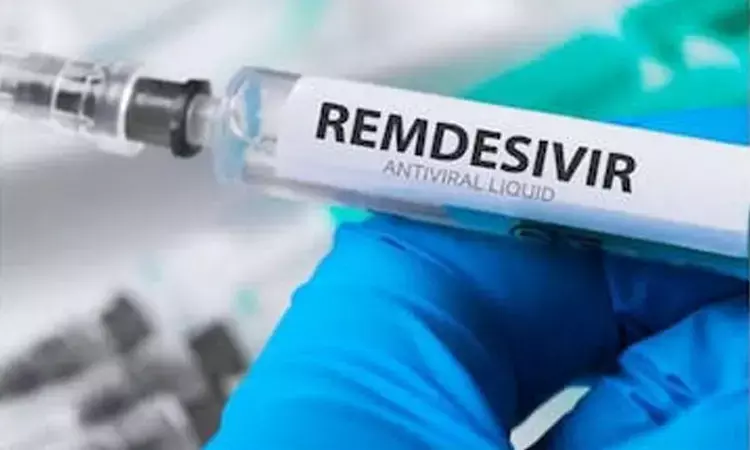
Written informed consent of each patient is required before the use of the drug while active post-marketing surveillance data and reporting of serious adverse events have to be submitted.
New Delhi: After Hetero and Cipla, another pharmaceutical major Mylan was given permission by India's drug regulator on Thursday to manufacture and market the anti-viral drug remdesivir for "restricted emergency use" on hospitalized COVID-19 patients, official sources said.
Written informed consent of each patient is required before the use of the drug while active post-marketing surveillance data and reporting of serious adverse events have to be submitted.
On June 21, Hetero and Cipla were given permission to manufacture and market the drug on the same conditions.
The Union Health Ministry in its 'Clinical Management Protocols for COVID-19' recommended the use of the drug in COVID-19 patients with moderate stages of the illness (those on oxygen support).
The drug has been included as an "investigational therapy" only for restricted emergency use purposes.
It is not recommended for those with severe renal impairment and high level of liver enzymes, pregnant and lactating women, and those below 12 years, the document stated.
The drug, administered in the form of an injection, should be given at a dose of 200 mg on day one followed by 100 mg daily for five days.
"The approval was given by the CDSCO on Thursday," an official source in the know of the developments told.
Mylan had already entered into non-exclusive licensing agreements with Gilead Sciences, which is the patent holder of the drug remdesivir.
US pharma giant, Gilead Sciences, had applied to the Indian drug regulatory agency, Central Drugs Standard Control Organisation (CDSCO), for import and marketing of remdesivir on May 29.
After due deliberations, permission under emergency use authorization was granted by Drug Controller General of India (DCGI) on June 1 in the interest of patient safety and obtaining further data.
On June 21, Hetero and Cipla were given permission to manufacture the drug. Besides, Jubilant, BDR, and Dr Reddy's Labs have also applied to CDSCO for permission to manufacture and market the drug in India and are still awaiting due permission.
Read also: Cipla to knock out Hetero Healthcare in pricing battle of Remdesivir?
Ruchika Sharma joined Medical Dialogue as an Correspondent for the Business Section in 2019. She covers all the updates in the Pharmaceutical field, Policy, Insurance, Business Healthcare, Medical News, Health News, Pharma News, Healthcare and Investment. She has completed her B.Com from Delhi University and then pursued postgraduation in M.Com. She can be contacted at editorial@medicaldialogues.in Contact no. 011-43720751


